
Led by senior engineers from Carlisle and Amphenol, our
product development, engineering, quality, operations
and sales teams are focused on meeting and exceeding the
unique requirements of our customers.
By
benefiting from a wide selection of connectors and
components ready-made for patient monitoring system,
Orantech offers significant savings for the customers on
OEM projects.
From Design Input to Final Assembly
At Orantech, our OEM cable manufacturing process is built around precision, partnership, and compliance. From initial engineering consultation through tooling, prototyping, and full production, we manage every stage in-house to ensure quality, traceability, and speed.
Our Shenzhen-based facility is ISO 13485:2016 certified, FDA registered, and equipped with state-of-the-art testing labs to validate performance and safety at each step.
Key Stages of Our OEM Manufacturing Process:
- Design collaboration & technical consultation
- Prototype development & performance testing
- Compliance review (510(k), CE, ISO)
- Final assembly, labeling, and QC
Why OEMs Trust Orantech:
- Full in-house production control
- Scalable for low- to high-volume manufacturing
- Support for regulatory documentation and audits
OEM/ODM Customized Service
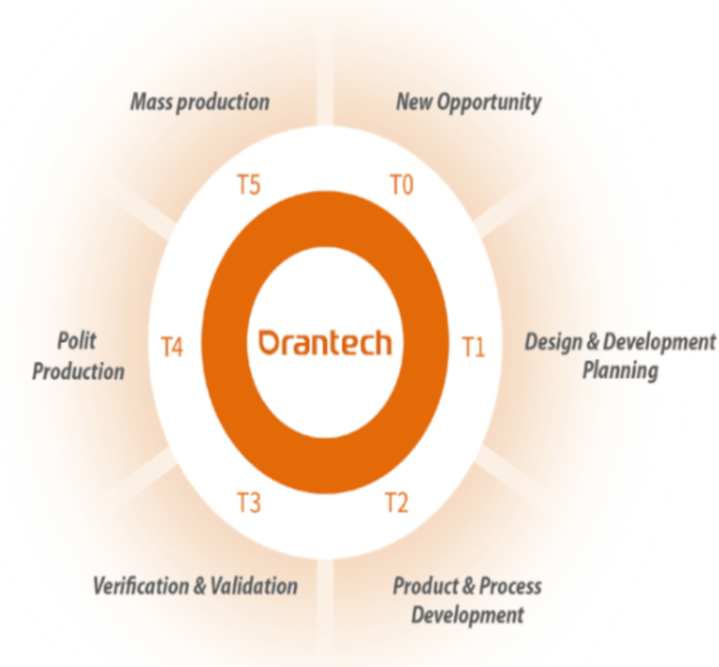
Our collaborative process involves six phases for targeted production:
Six Phases
T0: New Opportunity
• Requirements Gathering
• Needs Analysis
& Assessment
• Stakeholder Alignment
• Quotation
T1: Design and Development Planning
• Verify Technical Specifications
• DFM
Review
• Stakeholder Alignment
•
Establish Specifications
• MVP Generated
T2: Product and Process Development
• Flow chart/PFMEA/SOP/QCP
• Prototype
•
DVT Plan (In-house/3rd Party)
• DOE &
PV Protocol (IQ/OQ/PQ)
• TMV Protocol
• Customer Approval
T3: Verification and Validation
• Equipment/Fixtures Qualification
•
Component Qualification (FAI)
• Component
Inspection Plan
• Report (DVT/TMV/PV/MVP)
• Customer Approval
T4: Production
• Training
• Assembly FAI
• Production
Approval
T5: Mass Production
• Ramp up Plan
• Change Control Management
(ECN/PCN)
• Cost Saving Proposal
•
Customer Approval
• Satisfaction Assurance


Development and design
Orantech works in partnership with its customers throughout the development process, bringing a breadth and depth of expertise and solutions to solve the most complex challenges. Orantech provides expert teams in design, engineering, quality, and project management to help you deliver innovative products to the marketplace.
- Electro-Mechanical Assemblies
- Connector, Contact and Cable Design
- Injection Molding, Over Molding and Insert Molding
- Mold Flow Analysis
- Testing and Verification
- Cleaning Requirements & Validation
- Product Qualification
- Labeling and Unique Device Identification
Manufacturing solution
Based in China facilities with FDA registration and ISO
13485:2016 certificated, Orantech offers wide options to
meet your specific requirements and support an extensive
range of customized product solution in high or low
volumes efficiently, from components and kits to design
and certification.
Injection Molding/Over
Molding/Insert Molding
Cable & Wire Harness
Assembly
Ultrasonic
Welding/Crimping/Soldering/Adhesive Bonding
Custom
Tooling for precise performance in production
In-process
Inspections to ensure superior quality build in
workmanship
Process Development/Fully Validation
(IQ/OQ/PQ)
Laser Marking and Labeling
Lot
Control and Traceability


Supply chain
Medical device manufacturing requires continuous
optimization of innovation, risk management, supply, and
cost. Safety, quality, on-time delivery, continual
improvement, and maintaining the effectiveness of our
business management system is achieved through
teamwork.
Purchasing Controls
Design and Change Controls
Value Analysis &
Value Engineering Process
Schedule Production
Vendor-managed Inventory
Measure Logistics
and Service Excellence
Process Validation/MSA
executed at vendor facility
Quality assurance
At Orantech, Quality Assurance is a critical part of the
process. Compliance, Customer satisfaction, and meeting
all applicable requirements is the responsibility and
commitment of all Orantech employees.
FDA
Registered Facility
ISO:13485:2016 Certified
CE
Mark and FDA 510K approved products
Compliant with
applicable National and International Regulations
In house Testing and Verification Process
Qualification and Validation
In house TMV
(GR&R) Process
Protocol and Report Approval
Continuous
Improvement through Plan-Do-Check-Act Methodology


Contract manufacturing services
When you partner with Orantech, you’re connected to an expert manufacturer with diverse resources to manufacture your product at any scale required. We incorporate a formalized program for tracking customer documents, revisions, and updates to ensure customer-authorized change control and unit-to-unit repeatability between production runs.
Manufacturing Process Definitions
- DFM = Design for Manufacturing
- MVP = Minimum Viable Product
- PFMEA = Process Failure Mode Effects Analysis
- SOP = Standard Operating Procedure
- QCP = Quality Control Plan
- DVT = Design Validation Test
- DOE = Design of Experiments
- PV = Process Validation
- IQ = Installation Qualification
- OQ = Operational Qualification
- PQ = Performance Qualification
- TMV = Test Method Validation
- FAI = First Article Inspection
- ECN = Engineering Change Notice
- PCN = Product Change Notification
